(Apologies for the double-email day, but it’s probably better for readership to get this out today rather than waiting until tomorrow. I wasn’t sure when the Today story would run, so I went ahead with the previous post, which in retrospect I should’ve saved for tomorrow…)
Like much of the Entirely Too Online community, I was oddly captivated by the saga of the stuck-together ceramic bowls this week:


So it was a real treat to get an email from Alex Portée, a reporter for the Today show website, asking for help understanding what’s going on. The final story went live Thursday morning, with quotes from me and Jim Kakalios (I can’t get away from that guy…), but as always what’s in the final piece is much less than what I said, so I thought I’d use the infinite space available to me here to expand on it a little.
There are basically three plausible explanations for the bowls getting stuck together. One is just a physical snag— the surfaces are imperfect in such a way that they go together and come apart easily in one orientation, but a slight twist locks them in place. In that case, getting them apart would just be a matter of rotating them back into the easy orentiation. It’s a bit like the “child-proof” caps on bottles of pills in that sense.
The other two both involve the small space that necessarily exists between the two bowls, and I’ll illustrate them schematically here:

One possibility is that the bowls were stacked while still slightly damp, and as a result ended up with a small amount of water filling the gap between them. Interactions between the water and the ceramic will then produce a force holding them together— pulling down on the inside bowl, and up on the outside bowl. Essentially, the water is a little “sticky,” which holds the bowls together in the same way that condensation from your breath can let you hang a spoon from your nose.
The other possibility is that the space between the bowls is filled with nothing. Or, at least, with less stuff than it ought to be. This could happen if they fit fairly snugly, and were put together while warm— when the warm air in the gap between them cools down, it takes up less space, creating a low-pressure zone between the two. In that case, the downward air pressure from the weight of the atmosphere above us— constantly pressing in with a force of 14 pounds per square inch (I know it off the top of my head in American units; do your own metric conversions) is not balanced by the upward pressure from the air gap, and it acts to hold them together. This is a version of the trick that lets you stick a postcard to a water glass and flip it upside down. That gives you a net force pressing the bowls together that would need to be overcome to separate them.
In both of those cases, the easiest solution is just to wait. The seal between bowls in either case is not going to be perfect— scientists who want very good vacuum conditions need to work really hard to make good seals— so eventually either air will leak in or the water will evaporate out. That will either remove the adhesive force from the water or equalize the air pressure forces, and allow the bowls to be separated. Both of these could probably be sped up slightly by putting the bowls in an oven (upside-down on a rack), but it might very well take hours of baking to get results.
A lot of people in the replies to the original tweet suggested attempting to use thermal expansion to fix this— floating the outer bowl in hot water to make it expand, and putting ice cubes in the inner bowl to make it shrink. I basically agree with the numerous other people who said that ceramic bowls are singularly bad candidates for this— they just don’t expand or contract very much for reasonable changes in temperature, and getting an extreme enough gradient between the two for it to work out would be difficult to manage without a significant risk of cracking the bowls.
There were also a lot of suggestions to use vibration from various sources— a clothes dryer, an electric toothbrush, a sex toy— to shake the bowls. This is more likely to solve the locked-in-place version of the problem— prolonged gentle shaking might eventally rotate the bowls into the configuration where they separate more easily. There were also a lot of calls for using suction cups (again, frequently associated with sex toys, because Internet) to pull them apart; that seemed a bit unlikely to me, as the force holding a suction cup to the inner bowl would essentially be the same air pressure force that might be holding the bowls together (“suction cups” stick to things because you squeeze the air out from under the cup, and then atmospheric pressure holds it against the surface until air leaks in). There’s some set of parameters that would thread the needle between the force holding the cup to the inner bowl being stronger than the force pressing the inner bowl into the outer, but it would be tricky. Pressure forces are exerted over an area, and the area of a suction cup that fits inside the bowl will necessarily be smaller than the area of the bowl itself, so you’d need a significantly better vacuum under the cup than between the bowls.
In the end, the solution basically ended up being “wait and see”:


I don’t think there’s any real way to say which of the force-between-bowls scenarios was the actual culprit, here. The important thing is that the bowls are apart, with only one small chip out of the smaller two. So now, we can all move on to whatever the next Entirely Too Online concern will end up being…
If you liked this, and want more of it in your inbox, well, click this button:
and than go do something vaguely physics-y and go viral with it. If you want to argue with my interpretations, or point and laugh at my rudimentary figure-making, the comments will be open:


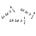

There is another explanation that occurs to me: A lot of lab chemists (like me) have learned the hard way not to store sodium hydroxide solutions in glass bottles with ground-glass stoppers, since a bit of basic solution between the stopper and the bottle can cause the two to chemically bond with each other. (Safety note: Trying too hard to resolve this situation can result in splashed hydroxide solutions and chemical burns.) Perhaps some basic chemical (in, maybe, dish soap?) promoted a similar bond?
Although we might not know the sticking mechanism, the nice thing here is that there is a resolution. Unlike, say, the "monolith" in the Utah desert, or mystery seeds arriving in the mail.